System evolution and biology
1. Biological systems
There are several indications that the system evolution
kinetics approach applies to biological systems. According to the general definition of systems considered, such biological systems can be any living entity (bacteria, cells, organs, mammals,...) but also sub-systems in the organization of a living entity (such as the immunitary system) and super-systems as group of living entities (species, etc.). The variation of the force of mortality with time can be expressed by l(t)=dE/dt with E(t)given by Equation E-1.
This corresponds to a modified Weibull distribution. Gompertz law is found back when the parameter b is made =0.
Let us just show here one example of using Equation E-1.
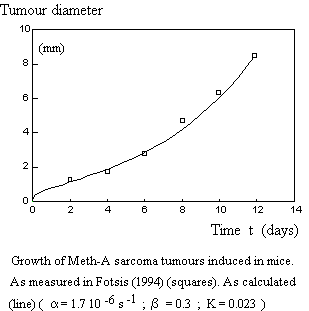
In addition, the General Adaptation Syndrome (GAS) described by Selye (1936) as a response to stress perfectly corresponds to the theory. We recall that in the GAS, one observes three successive stages: (1) adaptation ; (2) steady state ; (3) exhaustion, exactly as for the evolution of systems. Clearly, the concept "stress" used by Selye meets the constraint S due to the operation of a system in a given environment.
An article on the subject has been published in an international journal (Crevecoeur,
2001). Following article goes in the same direction : A mechanical system
interpretation of the nonlinear kinetics observed in biological ageing.(2000)
Recently, the editorial text entitled Power of a Complex Systems Perspective to Elucidate Aging in the special issue of the Journals of Gerontology, Series A Volume 79, Issue 10, October 2024 proposed to use the systems approach as a new paradigm to elucidate biological aging. This is not new as I already used this approach 25 years ago as can be seen from both a. m. articles of 2000 and 2001.
Consequently, I wrote a letter to the editors of the Journals of Gerontology to draw their attention on this fact (the odd thing about that is also that in a.m. editorial text no reference is made to my articles ! So, in our times of instantaneous world information with Internet and ChatGPT, ... these articles appear to have been out of look ! Isn't it in a way funny ?).
The Editor in Chief of the J of Gerontology Medical Sciences, Dr. Lew Lipsitz, who edited this Special Issue, kindly answered as follows:
"I echo Dr. Duque's congratulations and thank you for sharing your foundational work in the science of complex systems. I enjoyed reading your perspective and am pleased that our special issue could build upon the concepts you described and apply them to important issues in aging. Your insights were ahead of their time." (March 18, 2025)
Many thanks for this answer, Dr Lipsitz !
2. Application to evolutionary dynamics
If biological entities can be considered as systems, then their evolution kinetics should not be contradictory to evolutionary dynamics in the Darwinian sense. Here we will give some tracks of thinking rather than to make a full demonstration.
2.1 Start of life on earth
Nei (1975) obtained a rough relationship between the number "n" of nucleotide pairs (bp) in the DNA of different species and their time of appearance on earth : n=n0.exp (a.t), with a = 2.3 10-9 years-1 for an estimated 3 109 years elapsed from bacteria to mammal (n0 is the initial DNA content, e.g., 4 106 bp for E.
coli). This relationship is equivalent to Equation E-1 with b=0.
Assuming life to exist on earth since 3.5 109 years (= zero time) and considering the appearance of (1) E. coli - 4 106 bp - after 200
millions years, (2) the first unicellulars, e.g., Neurospora - 4 107 bp - after 1.2 109 years, (3) the bony fish - 9 108 bp - after 2.6 109 years and the first mammals - 3.2 109 bp - after 3.1 109 years, one can compare the results using Nei's equation and Equation E-1. Both equations fit the points as seen on the figure.
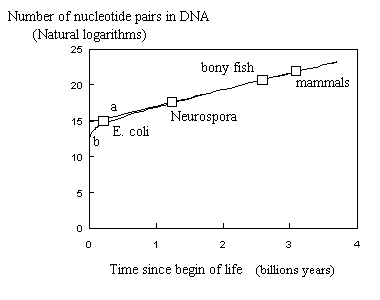
Fig. : Number of nucleotide pairs in genome as a function of time
from begin of life on earth. (a) As calculated with Nei's equation.
(b) As calculated with Equation E-1 (a=2.3 10-9years-1;
b= 0.45 ; K =
5.755 ). Squares correspond to 4 selected species from Nei.
However, as can be seen from the beginning of the curves (see following figure), extrapolating to point zero gives a figure of above 2 106bp ( 2,525,134 bp) for Nei's equation.
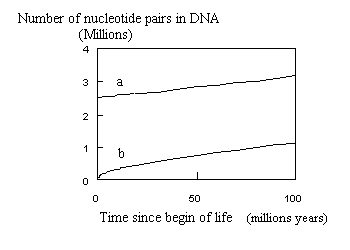 Such a big number of nucleotide pairs per genome seems unrealistic directly at start of life. On the contrary, using Equation E-1 allows to start from small molecules (0 bp at time 0; 229 bp after 1 year; etc.)
Such a big number of nucleotide pairs per genome seems unrealistic directly at start of life. On the contrary, using Equation E-1 allows to start from small molecules (0 bp at time 0; 229 bp after 1 year; etc.)
Whatever the model, the curve must start from zero. With Equation E-1 and b=0.45one better understands why the increase was so quick in the beginning (see remark of de Duve) and slowed down afterwards.
2.2 Feedback loops and evolutionary dynamics
Above considerations make sense, as we can re-interpret Figure 1and Figure 2 in the scope of evolutionary dynamics. Indeed, while Figure 2 corresponds to the environmentally induced changes in living organisms, e.g., genome mutations, Figure 1 is a good scheme for the adaptation of the fittest. The struggle for life in a limited environment will advantage the individuals with the best adaptation solutions. It has been demonstrated that advantageous mutations at a given locus can happen in specific stress conditions (environmental changes) much more often than in neutral conditions (Cairns et al., 1988 ; Hall, 1990). The positive feedback loops corresponding to an increased number of events (defects, mutations, etc.) in reaction to the environmental challenges induce adaptative efforts within the system (negative feedback loops), which can lead at a critical level to the emergence of a new behaviour or entity. This enlightens why ageing curves are applicable to evolutionary dynamics.
2.3 Step-by-step evolution
However, strongly advantageous solutions take time to become generalized to a whole population. So, the process will not occur in a continuous manner, but rather step by step. This is visualized in next figure.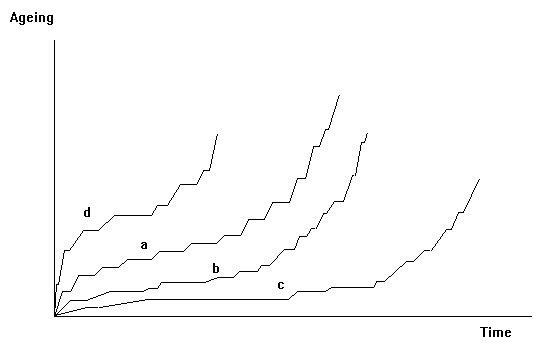
Curve "a" will correspond to a regular evolution, curves
"b" and "c" to slow evolutions in the beginning with some long periods without
apparent evolution, while curve "d" would be typical for a rapid evolution in
the beginning (e.g., due to severe challenges), which will end in a shorter
duration of the whole process.
Probably what is known as the "cambrian big bang" or the "cambrian explosion" of number of species is a step of exceptionally high amplitude which occurred in the development of life. Steplike evolution curves are what one encounters in nature when the attention is focussed on particular time intervals. Equation
E-1 is the envelope over all time intervals of a given process. Steplike life curves are in perfect agreement with Eldredge and
Gould's theory of "punctuated equilibrium" (1972). It is interesting to draw a parallel with the experimental work of Lenski and coworkers (Lenski and Travisano, 1994 ; Elena et al., 1996). These teams followed evolutionary changes in bacterial populations (E. coli) propagated for 10,000 generations in identical environments. They first showed that cell volume and relative fitness (to the ancestor) grew according to a concave (from below) curve (Lenski and Travisano, 1994). Such curves are similar to the first part of Figure 3. But, focussing on a population at a smaller time scale (3,000 generations), they got a better fit with a step model, what they considered to show evidence of punctuated equilibrium (Elena et
al., 1996). This confirms our interpretation of the ageing curve as the large scale envelope of evolutionary dynamics through punctuated equilibrium (compare Figure 3 to Figure 3.1 and Figure 3.2). It is thus a way to reconcile the followers of Gould and Eldredge with those who see evolution as a continuous process: the difference is in the time scale considered. Thus the adequacy of the chosen time scale is a key element for correct interpretation in evolution dynamics as it is in investigations on ageing.
Back to
Home page

Page last revised: April 6th,
2025
Best viewed with Explorer
© All rights reserved
- Sysev (Belgium) 15/03/98





